



Inorganic fluorine products
Aluminium fluoride for industrial use
Domestic product consultation
0086-15366800998
Foreign Trade Hotline
0086-15366800998
Product Details
Product Name: Aluminum fluoride
Abbreviation: AlF3
Synonyms: F luoridhlinity;ALUMINUM TRIFLUORIDE;ALUMINUM FLUORIDE;ALUMINUM(III) FLUORIDE
C AS :7784-18-1
Molecular formula: AlF3
Molecular w eight:83.98
Einecs:232-051-1
Dangerous goods transport number : non-dangerous goods
Structural formula :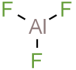
BRIEF DESCRIPTION
Aluminum fluoride is an inorganic substance with the chemical formula AlF₃, colorless or white crystal. Insoluble in water, acid and alkali. It is very stable and can be hydrolyzed by heating. Mainly used for aluminum smelting.
TECHNICAL INDICATORS
Analysis | Requirement |
Appearance | White powder |
F , % | ≥60 |
Al , % | ≥31 |
SiO2 , % | ≤0.32 |
Fe2O3 , % | ≤0.1 |
SO42- , % | ≤0.6 |
P2O5- , % | ≤0.04 |
Reduction on ignition | ≤1.0 |
Purpose (USE)
1. Aluminum fluoride is mainly used in aluminum smelting production to lower the melting point and improve the conductivity of the electrolyte.
2. Aluminum fluoride is used as an inhibitor of secondary fermentation in alcohol production.
3. Aluminum fluoride is used as a flux in ceramic glazes and enamel glazes and as a component of glazes.
4. Aluminum fluoride can also be used as a flux in smelting non-ferrous metals.
STORAGE CONDITIONS
1. Store in a cool, ventilated warehouse.
2. Keep sealed and away from children.
3. It should be stored separately from acids to prevent it from reacting with acidic substances.
4. Store separately from food chemicals and avoid mixed storage.
5. Suitable materials should be available in the storage area to contain spills.
PACKAGE
1. Plastic woven bags, aluminum foil bags, cardboard drums;
2. Other forms of packaging according to customer needs
CHEMICAL PROPERTIES
1. aluminum fluoride is heated with liquid ammonia or concentrated sulfuric acid, or eutectic with potassium hydroxide.
2. Aluminum fluoride is not reduced by hydrogen, does not decompose but sublimates under strong heat, and is very stable in nature. When heated to 300-400°C, it can be partially decomposed by water vapor into hydrogen fluoride and aluminum oxide.


Plant Evironment