



Inorganic fluorine products
Calcium fluoride
Domestic product consultation
0086-15366800998
Foreign Trade Hotline
0086-15366800998
Product Details
Product Name: Calcium fluoride
Abbreviation:CaF2
C AS :7789-75-5
Molecular formula:CaF2
Molecular w eight:78.07
Einecs:232-188-7
Dangerous goods transport number: non-dangerous goods
Structural formula :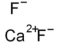
BRIEF DESCRIPTION
Calcium fluoride is the main component of fluorspar, or fluorite, and is a colorless cubic crystal or white powder. Glows when heated. It is soluble in concentrated inorganic acids and decomposes to release hydrogen fluoride. It is slightly soluble in dilute inorganic acids and almost insoluble in water. Relative density 3.18. Melting point 1402℃. Boiling point 2500℃. Low toxicity.
TECHNICAL INDICATORS
Apperance | White powder | |
Assay | % | ≥99.90 |
Iron(Fe) | ppm | ≤5 |
Cuprum(Cu) | ppm | ≤2 |
Chromium(Cr) | ppm | ≤1 |
Nickel(Ni) | ppm | ≤1 |
Manganese(Mn) | ppm | ≤1 |
Cobalt(Co) | ppm | ≤1 |
Weight loss on burning | % | ≤1 |
Purpose (USE)
Calcium fluoride , used to make hydrofluoric acid. The pure product can be used as a catalyst for dehydration and dehydrogenation reactions. Metallurgical flux. Optical glass. Polishing agent for electronic materials.
STORAGE CONDITIONS
1. It should be stored in a ventilated, dry and clean warehouse, sealed and protected from moisture.
2. Do not store or transport together with acids and foods.
3. Protect from rain and sun during transportation.
4. Be careful when loading and unloading to prevent packaging damage.
PACKAGE
1. Woven bag lined with plastic bag, net weight 25kg
2. Other forms of packaging according to customer needs
CHEMICAL PROPERTIES
Calcium fluoride has a relative density of 3.18, a melting point of 1423°C, and a boiling point of about 2500°C. The solubility in water is extremely small. At 18°C, only 0.0016g is dissolved in 100g of water. It is insoluble in acetone, but soluble in hydrochloric acid, hydrofluoric acid, sulfuric acid, nitric acid and ammonium salt solutions. It has almost no effect on dilute strong acids and hot concentrated sulfuric acid. The reaction generates hydrofluoric acid, which forms a complex when dissolved in aluminum salt and iron salt (Fe3+) solutions.
Plant Evironment