



Customized products
Lithium carbonate
Domestic product consultation
0086-15366800998
Foreign Trade Hotline
0086-15366800998
Product Details
Basic Information:
Product Name: Lithium carbonate
Abbreviation:Li2CO3
Synonyms:Camcolit, Candamide, Carbolith, Carbolithium, Carbolitium, Carbonic acid lithium salt (Li2CO3), Ceglution
CAS:554-13-2
Molecular formula:Li2CO3 [Customs Code]: 28369100
Molecular weight:204.3833
Einecs:209-062-5
Structural formula: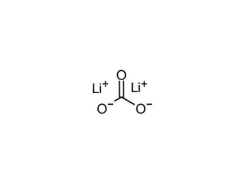
Brief description:
Lithium carbonate is an inorganic compound with the chemical formula Li2CO3 and a molecular weight of 73.89. It is a colorless monoclinic crystal, slightly soluble in water and dilute acid, but insoluble in ethanol and acetone. Its thermal stability is lower than that of carbonates of other elements in the same group in the periodic table. It does not deliquesce in the air and can be obtained by adding sodium carbonate to lithium sulfate or lithium oxide solution. Its aqueous solution can be converted into an acid salt by passing carbon dioxide into it, and hydrolysis occurs when it is boiled. It is used as a raw material for ceramics, glass, ferrites, etc., for spraying silver paste on components, etc., and is used in medicine to treat depression. Technical indicators:
Analyze Project | Analysis | Requirement |
Appearance | Appearance | Colorless monoclinic crystal |
density | Densities | 2.11 g/cm³ |
Melting point | Melting point | 720℃ |
Boiling Point | Boiling point | 1342℃ |
purity(%) | Fineness | 99.8% |
use:
1. Lithium carbonate can be used in the manufacture of lithium compounds, enamel and glass. It is the raw material for the production of lithium compounds and metallic lithium and can be used as an electrolytic bath additive for aluminum smelting. It is widely used in industries such as glass, ceramics, medicine and food. It can also be used in synthetic rubber, dyes, semiconductors, military defense industry, televisions, atomic energy, medicine, catalysts, etc. It is used to produce acoustic grade single crystals and optical grade single crystals. It can also be used to treat manic psychosis and make sedatives.
2. Battery-grade lithium carbonate is mainly used to prepare lithium-ion battery positive electrode materials such as lithium cobalt oxide, lithium manganese oxide, ternary materials and lithium iron phosphate. The main raw material of lithium batteries is battery-grade lithium carbonate. With the advancement of new energy technology, progress and breakthroughs have also begun to be made in the field of transportation. Among them, lithium-ion batteries are the core energy of new energy vehicles and have played a decisive role in the development of new energy vehicles. The use of battery-grade lithium carbonate to prepare lithium batteries has better quality and performance, can reduce interference from the outside world, thereby extending its service life and increasing the actual endurance time, which plays a vital role in the development of the new energy vehicle field.
3. In pharmacy, lithium carbonate can inhibit mania and has a good improvement effect on schizophrenia and affective disorders. And in the actual application and treatment process, it will not have a negative impact on the mental activities of normal people. Therefore, in actual clinical applications, it is often used in the treatment of bipolar disorder. In pharmacology, it mainly stabilizes serotonin to inhibit symptoms, and plays a positive role in the field of medicine. In addition, it also has a good therapeutic effect on the treatment of diseases such as excessive menstrual bleeding and uterine fibroids.
Storage conditions:
1. Avoid rain and water, and avoid direct sunlight.
2. Store in a sealed container in a cool, dry and ventilated place.
3. It should be stored separately from oxidants, acids and fluorine, and should not be mixed.
4. The storage area should be equipped with appropriate materials to contain leaks.
Package:
1. Three-in-one composite bag lined with plastic bag, or fiberboard barrel.
2. Other forms of packaging can be provided according to customer needs.
Chemical properties:
1. Solubility: Lithium carbonate is slightly soluble in water and has a high solubility in dilute acid, but is insoluble in ethanol and acetone. In aqueous solution, lithium carbonate can be converted into acid salts and hydrolyzes when boiled.
2. Thermal stability: When heated to boiling point, it begins to partially decompose into lithium oxide and carbon dioxide.
3. Toxicity: Under normal circumstances, lithium carbonate is stable and non-toxic. However, under high temperature or light exposure, lithium carbonate will decompose and may release toxic carbon dioxide.
photo:

Plant Evironment