


Customized products
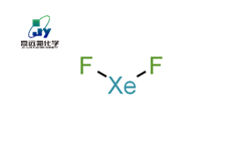
Xenon difluoride (XeF2)
Domestic product consultation
0086-15366800998
Foreign Trade Hotline
0086-15366800998
Product Details
Xenon difluoride (XeF2), also known as xenon difluoride, with a molecular weight of 169.30, is a colorless solid that easily sublimates at room temperature to form transparent crystals. Xenon difluoride decomposes in neutral or alkaline solutions, is relatively stable in acidic solutions, and has a pungent odor in aqueous solutions. Xenon difluoride vapor is colorless and has a nauseating odor. In the electronic industry, XeF2 is often used as an etching gas for silicon. Due to the spontaneous chemical reaction between XeF2 and silicon, XeF2 has a very high selectivity for the corrosion process of silicon. During the corrosion process of silicon by XeF2, the XeF2 gas diffuses freely to the surface of the silicon substrate and reacts with the outermost silicon atoms to generate gaseous Xe and gaseous SiF4, thereby achieving corrosion of silicon.
CAS :13709-36-9
Product Usage:
In the electronics industry, XeF 2 is often used as an etching gas for silicon. In addition, xenon difluoride is a highly selective fluorinating reagent with wide applications in the preparation of inorganic fluorides and organic synthesis. However, it only has fluorinating properties in gas-phase and non-aqueous solutions, and can only act as an oxidant in aqueous solutions.
Packaging and storage:
Xenon difluoride is a stable compound that can be stored for a long time in nickel containers or seamless aluminum alloy steel cylinders. The packaging specifications include 100g, 500g, 1kg, 2kg, and 5kg to meet the different requirements of research institutes and production enterprises. The specific packaging specifications can be customized and changed according to user needs. Xenon difluoride should be stored in a cool and ventilated warehouse, separate from oxidants, edible chemicals, and alkali metals, and away from sparks and heat sources.
Plant Evironment